Development and testing of a therapeutic pet food
Formulated or commercial diets for dogs and cats have been around for well over 60 years in North America. The first commercial diets were very similar in ingredient mix to the beef and raw foodtype diets mixed with grain and vegetables seen today as well as the large array of homemade recipes available from countless authors on the personal feeding of pets.
However, the production of commercial diets has come a long way in this time. Similarly, the research and development of pet nutrition has also advanced. As a result, modern pet foods are now more complete and balanced than human diets. In addition, most commercial pet foods are formulated and tested in a relatively prescribed manner. In contrast, therapeutic pet foods may be very different in formulation, nutrient profile and testing than regular commercial diets. The purpose of this article is to outline the kind of trials and testing applied to a therapeutic diet and to point out differences from regular pet foods.
Formulation: nutrient profile
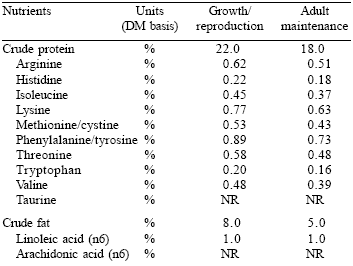
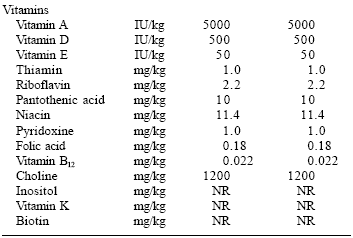
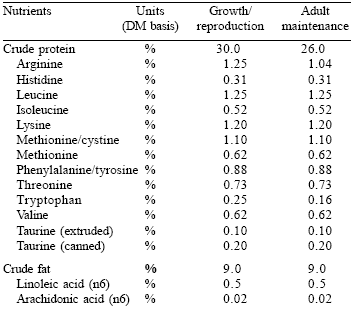
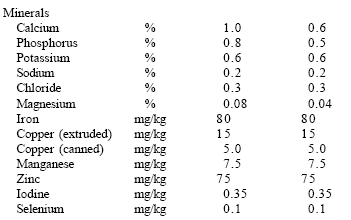
Most commercial pet foods are formulated to meet the nutrient profiles or standards set by the Association of American Feed Control Officials (AAFCO, 2003) (Tables 1 and 2). However, in therapeutic pet foods, it is usually necessary to meet a different nutrient profile than that recommended by AAFCO. For example, a formula designed for the dietary management of kidney disease would have a much lower phosphorus content, a lower protein level and by necessity a lower protein:calorie ratio than a commercial life stage diet.
Similarly, the B vitamin and zinc levels of a renal disease management formula would be significantly higher than that of most commercial diets due to excessive losses of these nutrients in urine. Thus, the nutrient profile of a therapeutic diet may be distinctly different from that of a commercial diet.
The formulation of a therapeutic diet would also be somewhat different than that of a commercial diet in terms of ingredient selection. A therapeutic type diet is selected with a more stringent set of quality assurance or quality control requirements.
Some ingredients, due to their inherent variability in quality or nutrient content, cannot be used in a therapeutic diet. Bioavailability of nutrients in a therapeutic diet would also be important. Selection of highly bioavailable nutrients such as proteinated minerals (e.g. Bioplexes) would be important.
Similarly, since the disease resistance of animals that require a therapeutic diet may be compromised, the addition of ingredients or nutrients to enhance immune function also becomes important (e.g. antioxidants such as ß-carotene, vitamin C, lutein).
The maintenance of a very specific dietary protein or phosphorus level becomes very critical in the formulation of a renal management diet. Thus, the quality control (QC) specifications for a therapeutic diet are also much different and more stringent than those of most commercial formulas. The selection of ingredients for most commercial diets are those approved by AAFCO. However, it is sometimes necessary to use ingredients in therapeutic diets that are different than those in the AAFCO listing in order to meet therapeutic objectives.
The nutrient content of a formula can be calculated on both an ‘as-is’ and a dry matter basis. This is sometimes used by commercial diet manufacturers in order to make the claim about satisfying the AAFCO Nutrient Profile. However, it is essential that a calculated nutrient content be verified by a specific nutrient analysis. This means that each diet must be analysed for each of the more than 40 essential nutrients.
| Table 1. The AAFCO (2003) canine nutrient profile. |
   |
Testing procedures for therapeutic diets
TRIALS DEFINING NUTRITIVE VALUE
AAFCO Maintenance Protocol
After the therapeutic diet has been formulated and analysed to determine its nutrient profile or content, the next stage of testing is the maintenance trials.
| Table 2. The AAFCO (2003) feline nutrient profile. |
  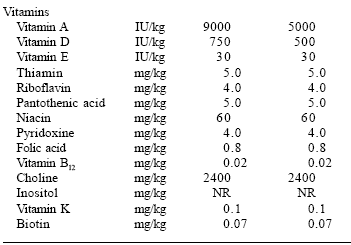 |
Although therapeutic diets differ from commercial diets in formulation, nutrient content and function, they must also satisfy the general requirements for commercial diets. In particular, all of the therapeutic diets developed by Veterinary Medical Diets (VMD) must satisfy the AAFCO Maintenance Protocol (AAFCO, 2003). This is a specific protocol in which the therapeutic diet is fed to a minimum of eight healthy adult cats or dogs (at least one year of age) for 26 weeks.
The therapeutic diet will be fed either ad libitum or based upon the energy needs of the cat or dog. All of the cats and dogs starting the trial must finish the trial and body weight must not decrease by more than 15%.
There are also specifications in relation to blood parameters. The dog must have at the end of the trial a normal haemoglobin, packed cell volume, albumin and serum alkaline phosphatase value. Cats must go through a similar trial with the addition of maintaining a specific blood taurine level (average 300 nmole/ ml). In addition to these parameters, VMD requires that each cat or dog have a starting and finishing complete blood chemistry analysis. If there are parameters that do not satisfy VMD’s requirements, then the formula may be rejected whether or not it passes the AAFCO trial.
In addition to AAFCO Maintenance Protocols, there are protocols for growth, gestation and lactation that may be used with the appropriate therapeutic diet. These AAFCO protocols may be conducted at independent testing facilities or inhouse research facilities.
Digestibility
In order to provide an accurate determination of the feeding rate for a specific diet, the metabolizable energy content (ME) must be determined. The ME of a diet can be estimated using the physiological fuel values of between 3.5-4.0 kcals per gram of carbohydrate and protein and 8.9 to 9.0 kcals per gram of lipid. However, this ME calculation is notoriously inaccurate, and does not provide any information about the bioavailability or digestibility of the diet. That is why it is essential that a therapeutic diet be subjected to a digestibility trial.
Using a total fecal collection protocol with a determined number of animals provides diet protein, lipid, dry matter, energy, ash and fibre digestibility coefficients. The energy digestibility can then be used to provide a relatively accurate determination of diet ME. For therapeutic diets, the protein, dry matter and energy digestibility must be in excess of 80%. The determined ME is then used in developing an appropriate feeding guide for the therapeutic diet.
Palatability
The formulation and analysis of a therapeutic diet may verify its potential as an effective dietary management tool, however, if the cat or dog will not eat the product, then the diet has failed. For this reason, it is essential that palatability trials be conducted to determine its acceptance by the cat or dog.
These trials are normally conducted at either independent testing facilities or in-house research facilities. These are normally two-day trials in which the diet is fed along with a similar or competitive product to 20 cats or dogs. The parameters measured include when animals first approach and first taste the diet as well as amount consumed. Observations are compiled and analysed statistically.
Palatability comparisons are usually made with other commercial diets to determine and maintain adequate palatability or acceptability characteristics.
EFFICACY TRIALS
In addition to the previously described trials and tests applied to commercial diets, therapeutic diets must undergo efficacy trials. Since each therapeutic diet is designed to produce or maintain a specific physiological response in the animal, then trials have to be conducted to verify this response. These trials are usually diet-specific.
For example, a diet designed to either control or prevent Feline Lower Urinary Tract Disease (FLUTD) must undergo a number of urine pH trials and a relative super saturation (RSS) test. The urine pH trial protocols are designed to determine responses in urine pH and specific gravity, blood pH, blood bicarbonate (blood gasses), etc. and must satisfy specific criteria established for these diets (Buffington, 1993).
The relative supersaturation determination is a measure of the concentration of certain compounds in urine of the cat or dog that would predispose the animal to either calcium oxalate or struvite formation. The RSS value is the quotient between the current ion activity product of a salt in solution and its activity product (Bartges et al., 1999). This study requires complete collection of urine over a 24-hr period. In addition to urine pH and RSS trials, other efficacy trials include:
• Weight reduction trials
• Carbohydrate digestion and blood glucose control
• Specific ingredient studies (eg. potassium citrate uptake and excretion)
On successful completion of efficacy trials, the therapeutic diet formula is ready for clinical trials.
It might be assumed that if the therapeutic test diet has successfully completed all of the preliminary and efficacy trials, the clinical trials should be a foregone conclusion. However, this cannot and should not be taken for granted.
CLINICAL TRIALS
The number of clinical trials conducted on therapeutic diets at Veterinary Medical Diets has been significantly increasing. It is safe to say that no new therapeutic diet would be launched into veterinary clinics without at least one successful clinical trial. Clinical trials are diet-specific and the clinical trial description and tests would also be specific or unique to the condition it is designed to support.
Case study: clinical evaluation of a feline therapeutic diet
The following is a brief description of a clinical trial conducted on a Veterinary Medical Diet product developed to dissolve struvite uroliths or bladder stones in cats (MediCal/IVD Feline Dissolution Formula).
The clinical trail lasted 12 months and involved the participation of approximately 50 veterinary clinics in Canada. Cats with clinical signs of struvite urolithiasis were selected on the basis of the following criteria:
• Radiographic confirmation of radiodense bladder stones
• Urine pH average greater than 6.4
• Detectable struvite crystalluria
Based upon the criteria, the cats were then placed on the diet (Feline Dissolution Test) as the sole source of nutrition. The cats were then monitored every 2 weeks, at which time the cats underwent the following:
1. Physical examination including body weight
2. Blood chemistry profile
3. Urinalysis
4. Culture and sensitivity of urine samples
5. Abdominal x-rays to determine if the stone had changed size
6. Abdominal ultrasound (in specific cases)
The Feline Dissolution diet was developed to be highly palatable, to produce a low urine specific gravity and maintain an acidic urine pH without inducing metabolic acidosis. The clinical trial determined that the Feline Dissolution diet completely dissolved the struvite bladder stone in an average of 21-42 days). Based upon these results, as well as the other tests and trials previously discussed, the product was then launched into the Canadian and more recently the American veterinary market.
Summary
The development and testing of therapeutic diets for cats and dogs are different from that of most other commercial life stage pet foods. The ingredients, nutrient profile, quality control and testing are more specific and unique to the therapeutic diet. The time period for testing of a therapeutic diet is also much longer than a commercial diet and would require two to four years to complete.
This article hasn't been commented yet.


Write a comment
* = required field