Animal feeding in Europe: challenges and opportunities
Animal nutrition in the European Union (EU) has been affected by several crises, such as the mad cow scandal (BSE), meat hormone residues, dioxin contamination, Genetically Modified Organisms (GMOs) and the concern about the use of antibiotics as performance enhancers.
Since the 1970s, animal production in EU has been under legal regulations aimed to enhance its safety and efficacy. However, during the last six years, issues such as BSE and others have put the sector in the mass media, which has made consumers less confident about consuming meat.
This paper will try to explain why and how the EU is dealing with the current subjects and also will describe sector strategies in place to recover consumer confidence. The intention is to answer some questions that today are worrying the animal production and feed manufacturing industries, such as: Is European animal production less safe than others? Why are the safety and legal regulations for animal nutrition more demanding than those for humans? Many are also wondering if the current opportunity (crisis) may produce a new model for European animal production. Finally, the current farming system will be discussed.
Consumer confidence in food safety and consumer perception
Today, food safety is an important global issue with international trade and public health implications. In this context, countries and also cultures perceive and handle food safety risks in very different ways
(Buzby, 2002). Safety risk assessments are complicated, and based on the ability to access and use science, detection technologies and mitigation methods. Countries, or even citizens of the same country with identical assessment processes, may perceive and handle the risk (management) differently. For example: Listeria monocytogenes (United States vs France etc.), consumer acceptance of irradiated meat (US citizens), GMOs (Europeans vs North and South Americans). European citizens are currently very sensitive to the issue of food safety. For instance, according to Bonny (2000), 32% of French citizens consider GMO a food safety concern, after BSE or ‘mad cow disease’ (Table 1). The same perception is identified in countries such as the UK and Germany.
Why are Europeans so concerned about GMO or biotechnology applications in food when the majority of scientific works indicate that there is no risk for consumers or the environment? One possible answer is that in the EU, agricultural technology does not benefit the majority of the population, as the number of people involved in agriculture in the EU is less than 4% of the population. A second argument would be that the new technology seems like threatening science fiction when presented by the mass media. Finally, GMO products are now financed by multinational corporations, and not by government funds as in the 1960s and 1970s when environmental activism and the Green Revolution started. All of these comments serve to enhance the misconceptions of consumers and increase the furor surrounding ‘feed scares’.
Table 1. Food safety risks of most concern to French consumers over the next 3 years (April, 2000).
Implications of recent European feed crises
The recent major food scares involving feed, such as mad cow disease, antibiotic resistance and dioxin contamination have resulted in new regulations. All of these new legislative regulations affecting animal
feeding have been implemented in order to enhance food safety and to restore the confidence of the European consumer in the food sector. This process is under the umbrella of ‘from farm to table’ or ‘from
table to farm’, a concept defining the European movement.
MAD COW DISEASE AND THE CONSEQUENCES OF THE BAN ON MEAT AND BONE MEAL
Mad cow disease (BSE) resulted in a large number of strong regulations on animal feeding within the EU such as the ban on meat and bone meal (MBM) and other animal by-products (e.g., plasma) for animal feeding. Beginning January 2000, vegetable materials had to replace MBM in animal feeds. European soybean meal production supplies 4% of the European requirements, and 78% of this is used by the animal feeding sector. Therefore, the ban affected the supply of protein for pigs, poultry and fish. According to the regulators, all of these measures are necessary to reduce the risk of cross contamination. For instance, today ruminant feed cannot be produced in a plant making non-ruminant feed containing fishmeal.
Recently, Rodehutscord et al. (2002) have reviewed the consequence of banning by–products from terrestrial animals in livestock feeding in Germany and the European Union. The review focused on the pig and poultry sectors. The main conclusions were:
1. MBM ban caused a need for alternative protein sources. Phase feeding with adjusted amino acid concentrations may solve the problem. Thus the ban is a minor problem in terms of ensuring amino acid supply.
2. Alternative plant ingredients cannot compensate for the lost phosphorus supply. Meat and bone meal once contributed 57% of phosphorus needed in animal feed, which presumably must be replaced by inorganic phosphates. General application of microbial phytases to all diets may reduce the amounts of mineral phosphates.
3. Energy from slaughter waste and mortality can be used in different technologies such as cement production.
4. Incineration or co-incineration of MBM and other by-products causes pollution due to gas emissions.
5. The effects of the ban of MBM on compound feed prices was not considered relevant. One kg of MBM not used represents an extra cost of €0.14.
Despite these conclusions, authors conclude that “if safety is considered from a hygienic point of view, MBM could considerably contribute to the goal of keeping limited nutrients within the nutrient cycle”.
Many people ask today if MBM and other byproducts might one day return to the European animal feed market. Actually some pending legislation may allow the utilization of MBM if refeeding and cross-contamination are avoided. However it would be difficult today to make this acceptable to feed manufacturing associations.
DIOXIN CONTAMINATION
More than 90% of daily dioxin intake comes from food (Malish, 2000), and products of animal origin are the principal source. The dioxin contamination of food is basically the result of contamination of the feed ingredients. Since 1997, batches of citrus pulp pellets, oils, fats and kaolin clay have been identified as contributing significantly to contamination of animal products with dioxin. The World Health Organisation (WHO) re-evaluated the allowable daily intake of dioxin in 1998 and established a tolerable daily intake of 1-4 pg TEQ/kg BW/day. WHO also stressed that the goal is to reduce the intake below 1 pg TEQ/kg BW/day.
In 2000, the Scientific Committee of Animal Nutrition (SCAN) adopted a scientific opinion indicating that dioxin contamination may occur at different levels with several chemical and technological origins. Fish oil and fishmeal from northern seas (Europe) are significant sources, and are much more contaminated than fish from the southern oceans (Chile, Peru). SCAN stated that more basic data would be needed in order to assess the impact of the contamination (See https://europa.eu.int/comm/food/fs/sc/scan/outcome_en.html).
HOW THE BAN ON ANTIBIOTIC ADDITIVES AFFECTED EU LIVESTOCK FARMING
In 1986, the Swedish government banned antimicrobial growth promoters (AGPs). Antibiotics and chemo-therapeuticals could only be incorporated in animal feed for alleviating or curing disease, not for growth or yield-promoting purposes. When Sweden joined the EU at 1995, the treaty of Adhesion to the European Union allowed this country to continue its ban for a period of four years, until the end of 1998. During this period of time, other Member States of the European Union (Denmark, Germany and Finland) took several steps against certain antibiotics (such as avoparcin, tylosin, spiramycin and virginiamycin), which until then had been authorised for use in animal nutrition for growth promotion purposes. The Scientific Committee for Animal Nutrition was consulted on these safeguard clauses.
Finally, the EU Commission banned the use of avoparcin in animal nutrition in 1997 and the EU Council of Ministers suspended the authorisation of tylosin phosphate, spiramycin, zinc bacitracin and virginiamycin as feed additives at the end of 1998. During the same period of time, the Scientific Steering Committee (SSC) of the European Commission addressed the question of resistance to antimicrobials. Its opinion on Antimicrobial Resistance was published in mid-1999 and is available at https://europa.eu.int/comm/food/fs/sc/ssc/out50_en.html. The SSC considered four ecological compartments for the transfer of resistance to antimicrobials: humans, animals, plants and soil water. The common factors among the four ecological compartments are antimicrobials, bacteria, and the genes that code for resistance. The SSC has since recommended several actions, which are carefully followed across the EU.
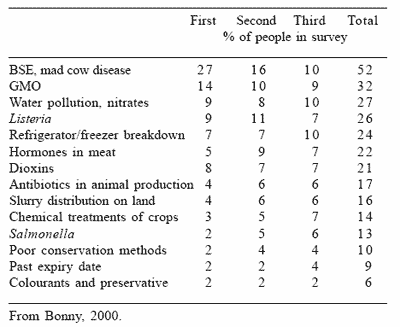
Concerning the process of monitoring resistance, Denmark has published the results of an annual surveillance study on the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans since 1997. From this surveillance it can be concluded that reduction in the use of antibiotics as feed additives decreased the incidence of resistance (Figures 1 and 2), DANMAP, 2001). The conclusion from the Danish surveillance was that the occurrence of resistance to avoparcin, virginiamycin and macrolides by Enterococcus faecium from broilers and pigs decreased during the five years following the ban.
A further advantage of the antibiotic ban should be an improvement in the perception by consumers of the use of products of animal origin, including meat, eggs, and milk. Also, the prohibition will build on a new strategy, which will be closer to ‘the green trend’ and the current ecological political climate. The disadvantage of the ban of AGPs is the marked increase in the cost of controlling subclinical diseases. This problem is particularly important when animals are housed under normal or poor management conditions. According to Jongbloed (1998), the ban of AGPs will reduce the efficacy of feed utilisation in the Netherlands between 3 and 8%. On commercial farms, where the standards of hygiene and management conditions are often not as high as those at research stations, the effect of AGP ban is even greater (Gropp et al., 1997).
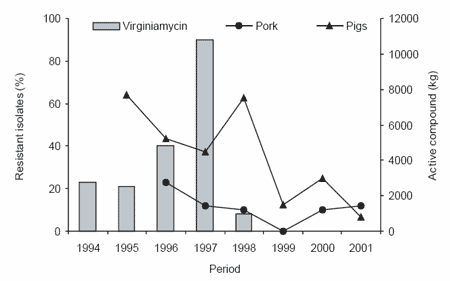
According to other studies, the ban will affect mainly piglet and broiler production, while laying hens and pigs in the growing and fattening period will be less sensitive. Hedegaard (2001) indicated that the
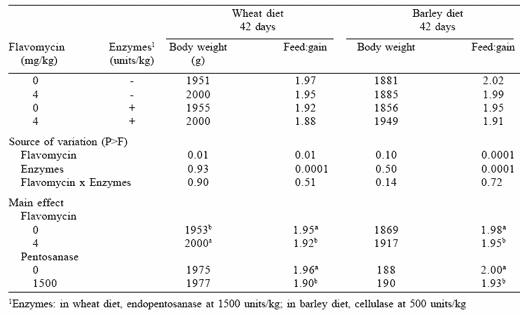
exclusion of AGP in feeds has resulted in an increase in antibiotic use in Denmark for treatment of piglets after weaning (Figure 3). The Danish Poultry Council statistical report, which included almost all of the commercial flocks in 1998, concluded that “the mean feed consumption at 42 days of age increased from 1.78 kg feed before the ban to 1.82 per kg of live bird after the ban and that it has remained higher than 1.81 kg” (DANMAP, 1998). Also, an increase in the number of flocks suffering from diseases related to Clostridium perfringens, such as necrotic enteritis and chronic hepatitis, was observed. In our laboratory, in chickens reared under ‘clean’ management conditions, Esteve-Garcia et al. (1997) have demonstrated that flavomycin, an AGP that has not been banned, at 4 mg/kg of feed significantly improved the feed conversion of broilers fed either barley or wheat-based diets (Table 2).
Figure 1. Trend in virginiamycin resistance among Enterococus faecium from broilers and broiler meat and the usage of the growth promoter virginiamycin in Denmark (DANMAP, 2001).
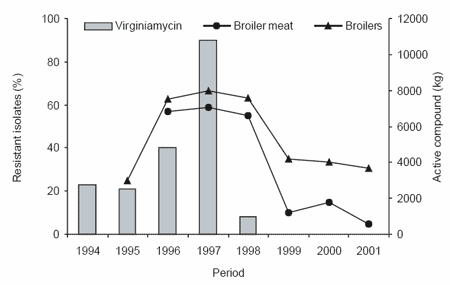
Figure 2. Trend in virginiamycin resistance among Enterococus faecium from pigs and pork meat and the usage of the growth promoter virginiamycin in Denmark (DANMAP, 2001).
In conclusion, the ban on the use of antimicrobials as feed additives will have a significant impact on livestock producers including higher incidence of 1) diarrhoea in piglets after early-weaning due to E. coli and swine dysentery; 2) wet faeces in broilers and other avian species reared on the floor because of necrotic enteritis produced by Clostridium spp. and 3) acidosis and parakeratosis in intensive beef cattle and subclinical coccidiosis in broilers and other poultry if the use of ionophore anticoccidials are no longer permitted in the feed.
Figure 3. Trend in usage of antimicrobials for treatment of food animals by route of administration in Denmark from 1996-2001 (DANMAP, 2001).
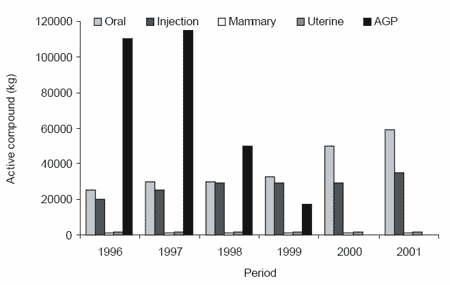
Table 2. Effects of flavomycin and enzymes on body weight and feed:gain of chickens fed wheat or barley diets (Esteve-Garcia et al., 1997).
Currently, the most intensive activity in the animal production sector in Europe is to find alternatives to antimicrobial growth promoters. Therefore, there is an intensive research initiated by the animal nutrition industry to discover new products.
How to compensate for the AGP ban at farm level: are there potential alternatives?
The SSC and many researchers (Piva and Rossi, 1999; Vanbelle, 1989; Mateos et al., 2001) have described alternative products and their potential action. There are three main practical tools by which the livestock producer and nutritionist may be able to overcome the ban on the use of AGP:
1) Improved management of animals.
2) Altered feeding programs and diet formulations.
3) Supplementation of diets with alternative additives.
Although all of these approaches may be considered effective, there is concern about how efficacy of new additives can be assessed. In fact, the efficacy assessment of probiotics is one of the most difficult
assessments according to SCAN.
THE MANAGEMENT OF ANIMALS
The management of intensive animal production under this ban situation should be one of the most critical points. Livestock producers must improve the quality of housing and rearing conditions, as animals without AGPs will be more sensitive to environmental pressures. For instance, the Swedish model has imposed animal welfare legislation that is highly demanding as to housing and level of management. The first aim of Swedish Animal Welfare (1988) is to prevent animal diseases through improved production methods. Several Swedish associations and pig producers compared the additional costs required to implement the Danish model, which at that time was considered to represent the EC directives (Ministry of Agriculture, Sweden 1998). The conclusion was that the additional costs for the ban on antibiotics including feed additives, straw or litter for sows and higher weaning ages in piglet production were compensated by better health, higher daily gain and improved feed conversion. The sum of added costs of the Swedish model was 0.51 SEK per kg of pig meat, which is 7% more than the current cost of pig production in Spain. The key question is, should consumers pay for this increased meat cost?
FEED COMPOSITION
Feeding programs and feed composition are important factors that predispose livestock to animal diseases, especially when AGPs are not included in the diet. Pluske et al. (1996; 1998a, b) in Australia
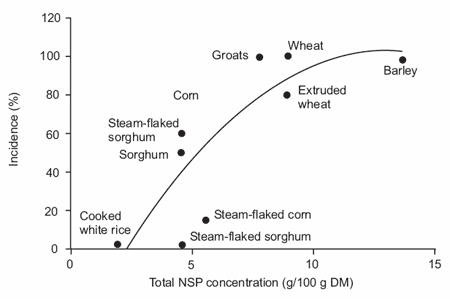
demonstrated that the clinical expression of swine dysentery (SD) following experimental infection with S. hyodysenteriae was markedly reduced when pigs were fed diets containing less than 5% total non-starch polysaccharides (NSP) (Figure 4). These results suggested that the soluble fraction of NSP (>1.0 g/100 g) predisposes pigs to swine dysentery. Furthermore, the incidence of disease in pigs fed diets containing a lower proportion of soluble NSP (<1.0 g/100 g) is affected by the concentration of resistant starch in the feed. Their findings are relevant since drugs that contain the imidazol group used in therapeutic control of SD or tylosin as a feed additive (preventative) have been banned.
In regards to poultry, Craven (2000) reported that the addition of rye in chicken diets increased the numbers of C. perfringens in the ceca and the number and frequency of recovery of C. perfringens in other segments of the intestine. Therefore, these findings confirmed the observation of Misir and Marquardt (1978) that penicillin supplementation of rye diets improved chick performance. Currently, several research groups have investigated the relationship between intestinal microflora and the role of water soluble NSP present in the diet (Choct and Annison, 1990; Hofshagen and Kaldusdal, 1992; Almirall et al., 1995; Langhout, 1998). In our department, Francesch et al. (1999) observed higher C. perfringens counts in ceca of birds fed rye compared to wheat- or barley-based diets. The high viscosity of digesta in rye-fed birds is one of the possible explanations because it causes a delay in the rate of passage of digesta resulting in a greater colonisation by microorganisms.
Figure 4. The relationship between the incidence of swine dysentery (%) and total NSP concentration of diets (y= - 57.97 + 26.85x – 1.10x2, R2 = 0.712, P = 0.002) in pigs fed different diets (Pluske et al., 1996).
FEED ADDITIVES: ALTERNATIVES TO AGPs
Currently there are many feed additives that have been shown to have beneficial effects on animal performance and in the control of subclinical diseases. These products can be divided into several categories including: 1) organic acids (formic, lactic, propionic and others); 2) enzyme preparations, including glucanases, xylanases, proteases, phytases and others; 3) microorganisms (probiotics); 4) oligosaccharides and others compounds (prebiotics), and 5) herbs and essential oils.
Although the majority of these products are considered GRAS (General Recognized As Safe), all must demonstrate safety and efficacy in order to be approved under the current European legislation. This means products authorized have presented a dossier covering aspects related to the protection of the end consumer, worker, target animal, environment and efficacy, following guidelines outlined in: https://europa.eu.int/comm/food/fs/sc/scan/out37_en.html and https://europa.eu.int/com/food/fs/sc/scan/out68_en.pdf.
The efficacy assessment of potential alternatives to AGPs is the real challenge, especially in the case of microorganisms, prebiotics and herbs or essential oils. In many cases it is difficult to prove efficacy in
animal performance in experimental facilities where the standard of management and animal heath are much better than at farm level. The implementation of new methods based on molecular biology may
mitigate this problem. However, methodologies described by Apalajalahti et al. (1998), based on the measurement of guanine + cytosine percentage in DNA of intestinal microbiota or other methodologies (RPLF) could provide useful information about the complexities of microbial ecology at the intestinal level and its role in the digestion process. Recently, some publications have shown modifications of intestinal flora in chickens fed different diet ingredient composition or challenged with coccidia (Apalajalahti and Bedford, 2000).
Legislative consequences of the crisis in food safety in Europe
Having gone through the crisis on food safety and especially the BSE scandal, the European Commission has taken some important decisions since 1996. The most important was to move the scientific safety risk assessment from the legislative body to a new and more independent division named Health and Consumer Protection. The Scientific Steering Committee has produced a very important ‘White Paper on Food Safety’, and the goal of this document is to assure the highest standards of food safety and to recommend establishment of an independent European Food Safety Authority (EFSA). The White paper proposed standards of food safety, which introduced an integrated approach to the responsibility of feed manufacturers, farmers and
food operators on the traceability of feed and food and their ingredients. At the same time, the steps in that approach needed to be transparent, making public the relevant scientific opinions and inspection
reports.
This new agency EFSA would provide scientific advice on all aspects relating to food safety, operate rapid alert systems, and communicate and conduct dialogue with consumers on food safety and health issues as well as network with national agencies and scientific bodies. EFSA initiated this operation in 2002 and it will be fully operative in May 2003. The potential scope of the Authority will be mainly to carry out the risk analysis, which comprises risk assessment, risk management and risk communication. All of these actions must be taken under independence, excellence and transparency .
Comments on two current questions about EU animal feeding
IS EUROPEAN ANIMAL PRODUCTION LESS SAFE THAN OTHERS?
After the recent scandals it would appear that European animal production and especially animal nutrition is not safe. There is considerable criticism of this, however the EU has a vast range of legislation that applies to feedstuffs, additives, vitamins, minerals and all substances which come into contact with food or feed during the manufacturing process. The EU decides which products are authorised in feed production and whether these substances pose a risk to human health if residues remain in the animal, edible tissue or affect the environment. This means that the EU legislation is based on criteria for use of specific products. Products are added to the positive (allowed) list after a scientific safety risk assessment. Independent scientific committees belonging to the Health and Consumer Protection division conduct the assessment which in future will be conducted under EFSA.
Once the scientific risk assessment is completed, the risk management is conducted by the legislative and administration bodies of the European Commission, which then become the responsibility of the public authorities in each EU country. Actually, thanks to the new regulations, it is thought that the health of the system is better than it once was and will result in bringing back consumer confidence in safety of animal production.
Despite the fact that EU animal production is under fire due to food safety scandals, standards of health and welfare of producing animals are among the highest in the world. Perhaps one explanation as to why consumer perception of EU-produced animal products is still low is because most of the EU actions have been adopted in the form of safeguard measures and the risk management and communication had some difficulties.
WHY IS THE SAFETY STANDARD AND LEGAL REGULATION FOR ANIMAL NUTRITION MORE STRICT THAN FOR HUMAN FOOD?
This question is frequently asked, because the risk assessment on feed additives and feed ingredients is much more intensive than for products added to food. Therefore, the idea of safety of feed additives
is not the same as that of food additives. Prof. G. Groop, member of SCAN (personal communication), justifies this inconsistency because nowadays consumers do not have the same opportunity to choose when faced with a canned food versus a cut of meat or other type of raw animal product. In consequence, the strongest regulation must be at the most basic level, which is animal feed formulation. For instance, today enzyme preparations and microorganisms for animal feeds that have already been approved for human food are submitted to a safety assessment superior to what is requested for human food.
New model for European animal production: from quantity to quality
European animal production will doubtlessly be different in the future. Farmers must look to consumer demands, and establish farming systems that recover public confidence in foods of animal origin as a first priority.
New farming systems must also be sustainable. According to Hartung (2000), this principle is understood to mean “conserving the existence of something in the long term, or adapting it without any disadvantage for humankind and nature. In the broader sense, this applies to farming as a whole and use of nature”. In addition, sustainability in livestock management must include not only environmental protection aspects, but also most particularly animal health and welfare. Safe animal food production will be another important criteria related to the sustainability concept. In conclusion, only high quality and safe food will be found in the market in the long term. In summary, the sustainability of animal production in the EU will rest on three main points: animal protection, consumer protection and environmental protection.
In consequence, will future European animal production remain intensive, specialised and regionally concentrated? Trying to answer this question, it would also be of interest to look to the proposal of Hartung (2000), who considers that at the present time there are three trends developing:
1. There is a trend towards a more simple and natural farming system, which can be interpreted as ecological animal farming. It means lower numbers of animals per land unit area, and special rules of implementation for use of organic fertilisers. Use of outdoor and extensive farming management is attractive in many ways, but it requires more land, causes more soil erosion, and requires more intensive animal health care.
2. There will be further technical developments in housing, animal identification and automation. The future will be characterised by the use of microchips and ‘intelligent’ machines. The advantages have been used only for the benefit of man to date. The novel development will be to create machines that enhance welfare. In conclusion, the use of new technology will give answers to the question of whether scientific applications can create an animal-friendly farming environment.
3. There is great focus today on the potential use of new biotechnological processes. Field reproductive biology and molecular biology can be used to improve many important aspects of animal production such as reproduction, improved diagnosis of hereditary defects, use of recombinant substances (somatropin, phytase or any other glycosidase enzyme preparation), etc.
Conclusions
Given these three developmental trends, it cannot be forgotten that the future will be defined by the consumer’s wishes. Perhaps European farming systems will be far different from what they look like today. However, sustainable animal production must employ biotechnology responsibly to increase the confidence of consumers, and the attitude of farmers and technical people will also be very important in order to improve the welfare of animals and the safety of the final products.
Having taken into account the potential developments in animal production, the future for agriculture looks to be intensive due to the high requirements of biotechnological inputs and specialised due to the great demands for technological knowledge. The current regionally concentrated animal production might be modified substantially for environmental reasons and animal health restrictions. Finally, the goal of European animal production will be to produce high quality, safe products acceptable to consumers, compatible with the environment and cost-effective for farmers.
This article hasn't been commented yet.


Write a comment
* = required field