Dietary protein – from amino acid supply to bioactive peptides
The word protein is derived from the Greek proteios, meaning primary or foremost. This is fitting in that proteins, being the molecular instruments through which genetic information is expressed (one gene – one enzyme), are central to the life process. There is a huge diversity of proteins. The key to the structure of different proteins is the group of relatively simple building block molecules, the amino acids. It is indeed remarkable that the cell can join what is a relatively small number of amino acids in many different combinations and sequences, yielding either peptides or proteins having strikingly different properties and activities. Over the years, research in animal nutrition has moved from describing the protein content of feedstuffs to the amino acid content, through to the absorption and metabolic utilisation of amino acids. Recently, attention has turned toward describing peptides that can be derived from food proteins during natural digestion. As is true for the remarkable diversity of protein types, there is also a plethora of these peptides, many of which have quite potent activity and significant biological roles.
In this chapter, I intend to draw largely upon results from my own laboratory at Massey University, New Zealand in an attempt to highlight developments in amino acid and peptide nutrition. Current understanding on how to describe the amounts of amino acids in diverse feedstuffs and their ‘availability’ to monogastric animals will be outlined, leading on to a discussion of bioactive peptides, which is an area that may very well herald a ‘new frontier’ in animal research.
Dietary amino acid supply
AMINO ACIDS IN FEEDSTUFFS
As late as the 1960s it was still common to find compounded diets for the growing pig formulated on the basis of total crude protein. With the more widespread use of chromatographic techniques, however, formulation on the basis of gross amino acid contents became more common, such that by the late 1970s diets were almost exclusively formulated with regard to gross amino acid content. The work of W.C. Rose and co-workers in the early 1950s led to the definition of essential (indispensable; must be ingested) and non-essential (can be synthesised in the body) amino acids. Consequently, much emphasis has been placed on the essential amino acids considered to be the most limiting in formulated diets.
More recently, however, the essential/ non-essential classification has been queried, as it does not allow for gradations of essentiality brought about by different physiological circumstances, and the distinction is highly dependent upon the criteria of evaluation. If, for example, maximum growth rate is taken as a criterion, then glycine and proline may be considered ‘essential’ for young avian species. Another example, is that cysteine may be considered ‘essential’ in the human infant as the trans-sulphuration pathway is poorly developed. These considerations have led to a proposed reclassification (Reeds, 1990) to include as many as four different categories of ‘essentiality’. Such a change in thinking has led to more studies into the individual properties of specific amino acids. This has led to major findings, such as the effect of tryptophan on food intake, the role of glutamine as a preferred gut metabolite, or the role for taurine in infant nutrition. As our understanding of the metabolic roles of individual amino acids increases, opportunities will present for nutraceutical solutions for both animals and humans.
Studies at my own Institute have recently centred on the sulphurous amino acid felinine, a unique metabolite found only in members of the cat family (Felidae). Curious as to the biological role for and the nutritional significance of felinine, we undertook to review current knowledge on the compound (Hendriks et al., 1995a) and to set up an HPLC analytical procedure. In attempting the latter, we found that several published chemical synthesis procedures for felinine actually produce an amino acid isomeric with felinine, but not felinine itself. This finding casts doubt over much of the published information on felinine and has opened the door for a re-definition of its metabolism and physiological roles. Moreover, a new high-yielding method for the synthesis of felinine (Hendriks et al., 1995b) has been developed. Our work has demonstrated (Figure 1) a sex-linked and very high excretion of felinine in the urine of the domestic cat. Such a rate of excretion has nutritional implications, with felinine excretion in the tom cat accounting for around 30% of dietary sulphur amino acid requirement. The presence or absence of this amino acid in urine can also be used to reassess phylogenetic relationships within the cat family. Some of our preliminary data are shown in Table 1.
There is currently much speculation as to a biological role for felinine. Perhaps it is involved in the regulation of sterol metabolism or it may have a pheromonal role. Our studies of felinine are on-going and seek to elucidate a biological function. Such information could lead to the development of new biotechnology products for the companion animal industry.
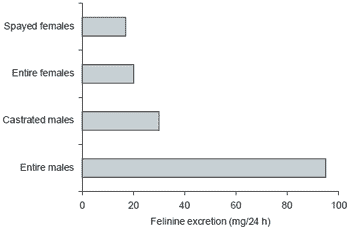
Figure 1. Mean 24 hr excretions of felinine in entire male, castrated male, entire female and spayed female cats (from Hendriks et al., 1995c).
Table 1. The detection of felinine in the urine of Felidae.
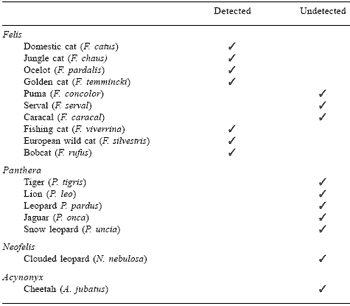
1Unpublished data, The assistance of the Auckland, Wellington, Melbourne, Adelaide, Antwerp, Rotterdam, Amsterdam, Rhenen, Cincinnati, Paris, San Diego and Singapore Zoological Gardens is acknowledged.
Advances in the chemical analysis of amino acids have been the subject of recent review (Williams, 1994; Rutherfurd and Moughan, 2000). While some refinements have occurred, the essential preferred methodology has not changed greatly over the last few decades, and during this time masses of information on the gross amino acid contents of feedstuffs have accumulated.
Such data sets have served to highlight the considerable degree of variation in amino acid content that exists even within well-defined feedstuffs. Figure 2 provides an example illustrating significant variation in the lysine content of soyabean meal, even when differences in crude protein content are taken into account. Also, and particularly for the amino acid lysine, it has been known for some time that when protein sources have been subjected to processing, conventional estimates of lysine content may considerably overestimate lysine present in the feedstuff in a chemical form that can be utilised by the animal (Tables 2 and 3). For such feedstuffs, assays such as Carpenter’s fluoro dinitrobenzene (FDNB) lysine have provided invaluable data on the ‘available’ lysine content.
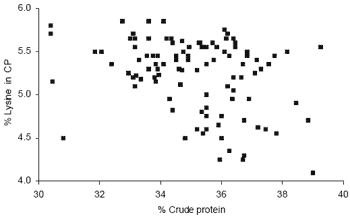
Figure 2. Soyabean meal lysine content in crude protein (% Lys in CP) (Adapted from Gill, (2000).
Table 2. Total lysine and ‘available’ (FDNB-reactive)1 lysine in 12 samples of meat and bone meal.

1Fluorodinitrobenzene lysine; Carpenter’s Method. – adapted from Moughan et al. (1989) with permission of the Society of Chemical Industry.
Table 3. Total lysine and ‘available’ lysine (FDNB – reactive)1 contents of soyabean meal extruded at different temperatures (T) and moisture levels (M).
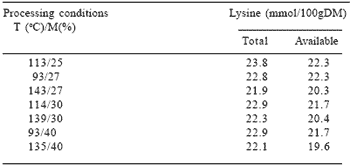
1Fluorodinitrobenzene lysine; Carpenter’s Method. – adapted from Hendriks et al. (1994).
Absorbed amino acids
Whereas the formulation of diets based on gross amino acid content was a major advance over crude protein, so too was a move in the early 1980s toward formulation based on digestible amino acids. Not only does amino acid content of feeds vary, but also amino acid digestibility varies, often quite markedly, both within and across feedstuffs.
Traditionally, amino acid digestibility measurement was based on analysis of the faeces or in the chicken, excreta. However, and because of the profusion of microorganisms in the hindgut that metabolise protein entering from the small intestine, faecal output does not accurately reflect the excretion of unabsorbed dietary and endogenous (of body origin) amino acids. It has been estimated (Mason, 1980) that 80% of the nitrogen present in the faeces of the pig is of bacterial origin. Similar estimates have been made in the chicken and other animals. In fact, faecal protein bears little resemblance to undigested dietary material.
The flow of amino acids at the terminal ileum is more representative of the unabsorbed dietary amino acid flow; and development of ileal digestibility measures has been another milestone in animal nutrition. Generally, faecal digestibility is considered to overestimate the actual digestibility of dietary protein in simple-stomached mammals and birds (Table 4). Moreover, ileal amino acid digestibility coefficients have been shown in a number of studies to provide suitably accurate data for the purposes of practical dietary formulation.
Little is known of the potential effect of microorganisms present in the upper tract on the derivation of ileal digestibility coefficients; and this is a topic urgently requiring investigation.
When dietary amino acid digestibility is determined at the end of the small intestine, it is important that correction is made for the endogenous amino acids, as endogenous protein makes up a sizeable proportion of the protein present in ileal digesta. Major strides have been made in the technology of determining ileal endogenous amino acid flows and these have been the subject of recent review (Moughan et al., 1998; Hodgkinson and Moughan, 2000). True ileal digestibility (whereby correction has been made for the endogenous component) is now well established as a preferred method for describing the uptake of dietary amino acids from the digestive tract.
Table 4. Comparison of the ileal and faecal digestibility of dietary protein for the chicken and several simple-stomached mammals.
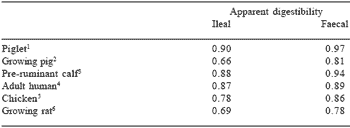
From Moughan and Donkoh (1991).
1Piglets (6 kg) fed bovine milk (Moughan et al. 1990).
2Pig (45 kg) given meat and bone meal based diet (Moughan et al. 1984).
3Milk fed calf (45 kg) (Moughan et al. 1989).
4Adult human (65 kg) consuming a meat, vegetable, cereal, dairy product diet (Moughan and Rowan 1989).
5Overall mean amino acid digestibility for 9 amino acids and 16 diets given to 10 week old chickens (Raharjo and Farrell 1984) and based on a collection of ileal digesta or excreta.
6Rat (80 g) given a meat and bone meal based diet (Moughan et aI. 1984).
A matter not widely appreciated is that for feedstuffs that have been subjected to processing or storage, (and with consequent chemical change of the constituent amino acids) the conventional ileal amino acid digestibility assay should not be expected to be accurate (Moughan, 1991; Moughan et al., 1991). This is well exemplified for lysine. During amino acid analysis whereby protein is hydrolysed with strong hydrochloric acid, early Maillard compounds are known to partially revert to lysine. Such reversion does not occur, however, in the animal’s alimentary canal during gastric digestion. Consequently, estimates of dietary and ileal digesta lysine will be in error leading to biased ileal digestibility coefficients. Although, and at least for lysine, structurally unaltered molecules can be accurately determined chemically (e.g. FDNB lysine assay), there is evidence (Hurrell and Carpenter, 1981) that the unaltered or chemically available molecules may not be fully absorbed from the damaged proteins.
The absorption (measured at terminal ileum) of reactive lysine has been determined in a recent study (Moughan et al., 1996) with the growing pig (Table 5). A casein-glucose mixture was heated to produce early Maillard compounds, and the amount of epsilon-n-deoxy-fructosyl-lysine (blocked lysine) and lysine regenerated after acid hydrolysis in the resulting material was calculated from the determined level of furosine. The amount of unaltered or reactive lysine was found by difference between the total lysine (acid hydrolysis) and regenerated lysine. The FDNB method allowed accurate assessment of the amount of chemically reactive lysine, which was grossly overestimated by conventional amino acid analysis (acidhydrolysed lysine), but the reactive lysine was not completely absorbed.
Table 5. Amounts of acid-hydrolysed lysine, FDNB lysine, reactive lysine and absorbed reactive lysine in a heated casein-glucose mixture.

1After conventional amino acid analysis.
2FDNB = fluorodinitrobenzene.
3Lysine units remaining chemically reactive after heating, determined from furosine levels.
4Reactive lysine absorbed by the end of the small intestine.
Moughan and Rutherfurd (1996) have developed a new digestibility bioassay to circumvent some of these problems. The new assay places emphasis on determining the uptake of chemically available lysine molecules from the gut, rather than the earlier preoccupation of trying to describe the uptake and utilisation of chemically altered lysine. With the new assay, the feedstuff (in its natural state) is fed to the test animal and samples of ileal digesta are collected. The reactive lysine in samples of the feedstuff and digesta are then determined after reaction with the agent o-methylisourea under controlled conditions by analysing the diet and digesta for the amino acid homoarginine. The true ileal digestibility of ‘reactive lysine’ is then calculated.
The new bioassay has been shown to be more accurate than assays based on conventional amino acid analysis as an indicator of digestible reactive lysine (Rutherfurd et al., 1997a). Overall for processed feedstuffs, digestible reactive (available) lysine will be overestimated using the conventional approach. (Table 6).
Table 6. Values for ‘available’ lysine (g/kg sample) in heat processed feedstuffs as determined conventionally or using a new (digestible reactive lysine) assay.
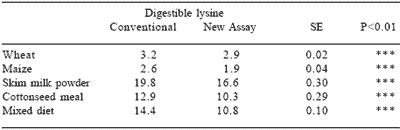
From: Rutherfurd et al., 1997b.
Over the years, much progress has been made in allowing a description in feedstuffs of the amino acids available for fuelling metabolism within the animal. Attention now needs to turn toward adapting these baseline assays to allow rapid, real time measurement of amino acid availability. In this way, considerable further cost savings can be secured.
Bioactive peptides – a potential new frontier in biotechnology
Recently, an exciting new era in protein research has emerged. This has come about from a realisation that during the digestion of proteins in the alimentary canal, variously sized peptides are released that have a wide range of physiological effects. Food proteins contain sequences of amino acids that are cleaved free during enzymatic digestion and then may be resistant to further breakdown. These peptides may act locally in the gut, or be absorbed and act systemically, to bring about a wide range of physiological effects. It is now known that there are many such peptides (often referred to as bioactive peptides) released during the digestion of food, but our understanding of these peptides and their effects is really still very limited.
Most research to date has investigated milk proteins and numerous bioactive peptides have been identified. Other food proteins, although not yet widely studied, are also known to contain biologically active amino acid sequences.
Bioactive peptides have been shown to elicit behavioural, hormonal, immunological, neurological, vasoregulatory and nutritional responses in animals. It has been clearly demonstrated that peptides generated from casein, lactoferrin and ß-lactoglobulin are able to effect a range of responses such as ACE inhibition (casokinins), antimicrobial activity (casecidin, lactoferricin), anti-thrombotic activity (casoplatelins), calcium absorption (caseinphosphopeptide) and immunomodulatory activity (casein, whey and lactoferrin hydrolysates). Moreover, effects on food palatability, gut function and the digestive processes have also been documented. Bioactive peptides are not only released naturally during digestion, but are also present in commercially produced protein hydrolysates. Thus there is a significant opportunity for the biotechnology industry to produce crude hydrolysates or semi-purified hydrolysate fractions that may be used to support more efficient animal production.
Commercial peptides (eg. casein derived phosphopeptides) are already being used as food supplements (Meisel, 1997a) and the pharmacological application of peptides has been investigated. As the research sector progressively screens the potentially enormous range of protein hydrolysates and unravels their physiological influences, there will be an opportunity for the biotechnology and feeds industries to capitalise on the knowledge generated and to produce innovative products. Early results are promising, and given the ubiquity of protein sources, the biotechnology industry faces a significant new frontier and what appears to be a major opportunity.
For the feeds industry, immune enhancement and antibiotic activity are properties of bioactive peptides that would appear to hold immediate promise for commercial exploitation. Documented effects on food palatability and the control of the digestive process are also highly relevant in practice but may present a somewhat longer-term opportunity.
The immunostimulatory and antimicrobial properties of various milk-derived bioactive peptides have been reviewed in detail (Meisel, 1997b; Xu, 1998; Clare and Swaisgood, 2000). A peptide from trypsin hydrolysed milk has been shown (in vitro) to stimulate the phagocytosis of sheep red blood cells by murine peritoneal macrophages and to enhance the resistance of mice to Klebsiella pneumoniae infection when given intravenously. A peptide from casein has shown protective action in mice against Staphylococcus aureus and Candida albicans. The same peptide has been shown to safeguard sheep and cows against mastitis when injected into the udder at levels comparable to those observed with standard antibiotic treatment. Current work from our own laboratory with rodents confirms the immunomodulatory activity of hydrolysates. Recently, Donnet-Hughes et al. (2000) have reported that Transforming Growth Factor (TGF-ß), polypeptides occurring naturally in milk, are effective in inducing remission and mucosal healing in patients suffering from Crohn’s disease.
Direct evidence in the literature that orally administered casein-derived casomorphins are able to regulate gastrointestinal motility, affect the rate of gastric emptying and exert anti-diarrhoeal action, has led our research group to investigate the effects of food derived peptides on digestive function in general. Already, our work has confirmed the earlier findings of others that the rate of gastric emptying can be influenced by the oral ingestion of peptides.
Moreover, and of considerable interest, have been the results from a number of experiments in the pig and other simple-stomached animals clearly demonstrating an effect of protein hydrolysates on overall gut protein dynamics as measured by the flow at the terminal ileum (ileal flow) of endogenous nitrogen or amino acids. It seems that dietary peptides influence gut protein secretory activity and/or endogenous amino acid absorption, leading to a substantial net increase in endogenous protein entering the large bowel from the small intestine. Such an effect has implications for dietary amino acid and energy requirements (leading to heightened requirements), to overall productive efficiency and to optimising digestive function.
Identification of the active peptides and the gut processes affected offers promise for means of manipulating the physiological processes at play.
Intriguingly it appears that the breakdown products of dietary proteins themselves may assist in regulating the digestive processes. The influence of dietary peptides on endogenous ileal amino acid flow is highlighted by the data presented in Tables 7 and 8. The results shown in Table 7 are from a study (Darragh et al., 1990) in which the endogenous ileal flows of serine, glutamic acid and alanine were determined in the growing rat given a semisynthetic diet where the sole source of nitrogen was either an hydrolysate of casein or synthetic amino acids. The animals also received a nitrogenfree diet as a control. The animals receiving the synthetic amino acid-based diet were in positive body nitrogen balance and the gut cells received a supply of dietary amino acids. In spite of this, the net endogenous amino acid flows at the end of the small intestine were no higher than those found for animals receiving a diet completely devoid of nitrogenous material.
However, the presence of dietary peptides in the gut brought about a very sizeable increase in net unabsorbed endogenous ileal amino acids. It is concluded that the peptides derived from casein have a major effect upon overall gut protein dynamics. It would appear that the peptides have a quantitatively significant influence on the secretory and absorptive processes.
The comparable results shown in Table 8 are from a similar study (Butts et al., 1993) in the growing pig and it is now also established that this effect of peptides is dose-dependent (Table 9). Clearly, given the quantitative importance of gut protein dynamics in overall body protein metabolism (Moughan, 1999), there are opportunities here to manipulate the gut processes. One can imagine, for example, enhanced digestive function arising from dietary peptide supplementation or significant improvements in growth efficiency brought about by a reduction in endogenous amino acid loss consequent upon removing or reducing the effects of key bioactive peptides.
Opportunities abound in what is an exciting new area of nutritional research.
Table 7. Mean endogenous amino acid flows (μg/g dry matter intake) at the terminal ileum of the growing rat.
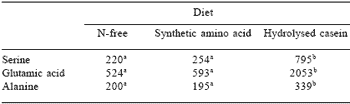
Means with differ ent superscripts were significantly different (P<0.05). Adapted from Darragh et al., 1990.
Table 8. Endogenous amino acid flows (mg/kg dry matter intake) at the terminal ileum of the growing pig given either a synthetic amino acid or enzyme hydrolysed casein-based diet.
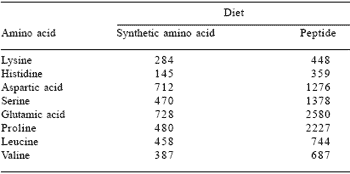
Adapted from Butts et al. (1993).
Table 9. Endogenous flows of lysine and total nitrogen (mg/kg dry matter intake) at the terminal ileum of the growing pig receiving purified diets containing graded levels of enzyme hydrolysed casein (EHC).

Adapted from Hodgkinson et al. (2000).
Quite in addition to their bioactive role, hydrolysates, and specifically peptides, are useful nutritional supplies of amino acids and are used in many human functional foods for this purpose alone. Recently, protein hydrolysates have become available as feed ingredients for animals, especially young pigs. These hydrolysates may have some specific nutritional properties. Firstly, the digestibility and availability of the amino acids in such hydrolysates is very high and there is unlikely (because the material is ‘pre-digested’) to be any limitation due to incomplete digestion and absorption. The uptake of peptides is based on a H+ -coupled mechanism, and if with the hydrolysates proportionally more (compared to intact protein) amino acid is absorbed in the peptide form, less energy may be expended by the animal in absorption.
Peptides from hydrolysates are known to be taken up into the portal blood more rapidly than the comparable intact protein, which may mean that there is less microbial degradation of key limiting amino acids. Finally, the commercial enzymatic breakdown of the protein and accompanying processing may reduce the amounts of active antinutritional factors known to be present in some plant materials.
Some preliminary production trials have been undertaken with hydrolysates as feed supplements for young pigs and these have shown promising results.
Studies recently conducted at Ohio State University and Michigan State University, reported by Tibbetts (2000) have shown that an enzymatic hydrolysate (of a non-animal protein source) can successfully replace spraydried blood plasma in the diets of young pigs. In one particular study, the hydrolysate supported better performance in comparison to a plasma protein control. In a further trial reported by Tibbetts (2000), conducted in the United Kingdom, the effect of replacing fish meal and full-fat soya by the hydrolysate was investigated.
Over the entire period of the trial (21 days) the performance of the pigs receiving the hydrolysate was superior, although the result did not achieve statistical significance. More information is required from controlled studies before definite conclusions can be drawn; but as can be expected, it does appear that hydrolysates have a valuable nutritional role. In addition to this nutritional role, the specific physiological/nutraceutical properties of peptides can also be exploited, which means that there is considerable potential for the development of new and superior value-added products. As our scientific understanding of bioactive peptides increases, more sophisticated and targeted hydrolysate products will be developed. The concept that a mixed feed provides more than nutrients, and in fact has functional/nutraceutical properties, will become more widely accepted in animal production, as is already the case in the foods industry. Products such as Alltech’s NuPro2000 is an interesting development. In addition to containing highly digestible protein and trace elements, it also contains peptides and nucleotides and contains a relatively high amount of glutamine.
This and similar products emerging on the world markets are pioneer functional foods for animals.
Conclusion
The ability to formulate diets in such a way as to supply the animal with a balanced array of available amino acids for body protein synthesis is central to efficient animal production. Over recent years technologies have been developed and refined such that this can now be achieved with a high degree of accuracy. A single remaining challenge is to develop analytical methodologies that are very rapid so as to allow a more flexible, dynamic, real-time approach to formulation. Research efforts are currently being directed toward this goal. Because of the central importance of amino acids in fuelling protein metabolism in the animal, much research has been centred on dietary protein from a nutritional perspective. However, the discovery of bioactive peptides has opened up a whole new field of study in relation to protein. There is potentially a very large number of bioactive peptides, and as new peptides are discovered and their activities become understood, there will undoubtedly be opportunities for pharmacological and nutraceutical applications within livestock production.
by Paul J. Moughan - Institute of Food, Nutrition and Human Health, Massey University
This article hasn't been commented yet.


Write a comment
* = required field