The importance of antioxidant protection: demonstrating and branding benefits in pet food
Animal nutrition in the last decade has seen great advances in the understanding and application of the correct balance of nutrients required in the diet and the quality of the raw materials used. In the companion animal sector, the focus on specific dietary requirements now means that pet owners have a huge choice of commercial diets of varying specificity and good nutritional quality available.
Often the quality of pet food is given the same status as human food, as it is bought using the same purchasing decisions as the family’s weekly groceries. Scares regarding the use of chemicals and drugs in animal feed have heightened consumer worries regarding the safety and health benefits of their pet’s diet. This, coupled with the rise in interest in feeding for animal well-being, longevity and preventative medicine, makes the role of dietary antioxidants increasingly important as a maintainer of health status as well as a potential marketing opportunity.
Recently consumers have become more concerned with the quality and ingredients that are used in both human food and animal feed. The idea that natural or organically derived ingredients can help us and our pets remain healthy is particularly attractive, as these components are seen as being, by inference, safe to use, whilst having multi-functional but nonpharmaceutical benefits. Antioxidants fit very well as natural products strongly associated with health benefits, and are regarded by many as vital ingredients in animal diets. There is a growing body of research evidence into how antioxidants work and which dietary sources are the most potent, which lends technical weight to the justification for using such ingredients in commercial production.
Importance of counteracting oxidation
Oxidation is a necessary phenomenon of cell functions as oxygen is utilised in respiration, metabolism and energy production. However, oxygen is highly reactive and potentially toxic (Knight, 1998); and fundamental mechanisms such as respiration result in the production of oxidizing compounds called free radicals or ‘reactive oxygen species’ (ROS), the majority being in the chemical form O2.
These are highly unstable and reactive and facilitate various chain reactions that damage cell membranes and tissue function. Free radicals have been identified as causative agents for pulmonary hypertension syndrome, inflammation, cancerous tumour development and reproductive disorders. Essentially, free radicals reduce cell function and interfere with cell growth by damaging DNA, proteins, lipids (and hence membranes) and carbohydrates, which is why antioxidant deficiency is linked to a variety of disorders.
Under normal, unstressed physiological conditions, between 3 and 5% of cellular oxygen is transformed into free radicals (Singal et al., 1998), an amount which increases dramatically under stress conditions. If the immune system is stimulated, free radicals are produced in large amounts during the proliferation of immune cells and can be used to damage and inactivate invading pathogens (Schwartz, 1996; Kettle and Winterburn, 1997; Surai, 2002), but will also cause further problems for the animal unless they are removed efficiently.
Preventing oxidation damage with antioxidants
Organisms have evolved specific antioxidant protective mechanisms that helped them to survive when oxygen concentration in the atmosphere was rising (Halliwell and Gutteridge, 1999). As a result there are thousands of naturally occurring compounds possessing antioxidant properties to disable free radicals. These compounds work in various ways, as shown in Table 1.
| Table 1. Description of antioxidant compounds and precursors (adapted from Surai, 2002) |
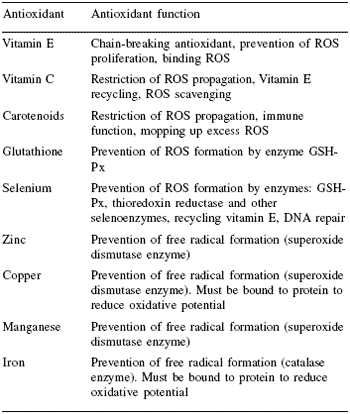 |
The main modes of antioxidant action can be split into three. The first is those compounds that prevent free radical formation in the first place. They do this by binding to co-factors required for formation of free radicals, such as iron. However, as is obvious from the immune example above, most reactions that generate free radicals are very important, so this is not always desirable.
The second mode of action is the ability to neutralise excess free radicals once they have been formed, as seen in compounds such as vitamin E, and prevent the initiation of cascade reactions. The properties of such antioxidants can be measured via a standard test known as a Trolox Equivalent Antioxidant Capacity (TEAC) analysis, where their chelating strength is measured against vitamin E in a standard free radical solution.
These compounds, however, can only bind a finite amount of free radicals, after which they become inactive. A constant dietary supply of antioxidant nutrients is therefore essential. This leads to the third mode of action, which is via the important antioxidant enzyme system in cells. These compounds work in concert with the chelating antioxidants by ‘recycling’ them – i.e. removing and neutralising the free radicals they have bound, reactivating the antioxidant. Two of the key enzymes involved in antioxidation are superoxide dismutase (SOD) and glutathione peroxidase (GSHPx).
Certain minerals are integral components of these enzymes and are required in sufficient amounts from the diet to ensure synthesis in cell organelles to maximise the efficiency of antioxidation. For example, GSH-Px synthesis is highly dependent on selenium availability. There are two major forms of SOD in the cell: manganese SOD, which is located in mitochondria, and copper or zinc SOD which is found in the cytosol. This enzyme transforms the O2 - free radical to form hydrogen peroxide H2O2 rather than the more reactive radicals such as hydroxyl free radical OH*, which would occur otherwise.
![]()
Hydrogen peroxide is more stable, but still toxic and must be removed from the cell by a further reaction, which is facilitated by either GSH-Px or catalase (CAT). Because GSH-Px is found in many cellular locations while CAT is located mainly in peroxisomes, the efficacy of H2O2 removal from the cell is greater with GSH-Px (Surai, 2002).
![]()
As this first level of antioxidant defence in the cell is not sufficient to completely prevent free radical formation and lipid peroxidation, therefore a second level of antioxidant defence includes fat-soluble (vitamins A, E, carotenoids, ubiquinols) and watersoluble (e.g. ascorbic acid, glutathione, uric acid) antioxidants. These antioxidants are potent chainbreaking compounds, which prevent free radical chain formation and propagation. This mechanism is not straightforward. For example vitamin E reacts with a lipid peroxyl radical (LOO*), the antioxidant is oxidised and lipid hydroperoxide (LOOH) is produced in the following reaction:
![]()
Lipid hydroperoxide products can react with metals such as iron to form cytotoxic products such as aldehydes, alkoxyl radicals (LO*) and peroxyl radicals (LOO*):
![]()
Again, GSH-Px is required to deal with lipid hydroperoxides.
Therefore selenium as an integral part of GSH-Px belongs to the first and second levels of antioxidant defence.
Even the second level of antioxidant defence is not able to prevent lipid peroxidation and some biological molecules are damaged.
In this case the third level of antioxidant defence deals with the repair of damaged molecules, consisting of specific enzymes such as proteases or lipases (Surai, 2002).
Whilst each level of antioxidant protection is important in its own right, it is only by ensuring they all work in harmony that complete protection can be afforded to the animal. It also ensures the efficient use of antioxidant resources that are often poorly stored in the body or in limited supply.
An example is the efficient recycling of vitamin E in the presence of sufficient vitamin C and selenium. Animal requirements for antioxidants vary with age, breed, health, work intensity, physiological status and environmental stress. Exposure to disease, air pollution (also cigarette smoke) generates oxidative stress. This makes it essential to supply adequate levels of antioxidant and precursor components for SOD, GSH-Px and catalase in forms that are readily taken up, and preferably stored in the body. When the oxidative stress level changes, tissue reserves leave the animal prepared to neutralise free radicals effectively before any serious damage occurs.
Antioxidant sources
Most antioxidants are delivered via the diet, either naturally occurring in raw materials or via supplementation. Fat-soluble antioxidants such as vitamin E and carotenoids operate in lipid-rich environments such as membranes, whereas watersoluble ascorbic acid (vitamin C) and glutathione, which are synthesised in the body, are found in the cell cytosol. All require adequate supplies of basic components for synthesis from dietary sources.
Deficiency (and, in some cases, excess) of these elements causes oxidative stress and damage to cellular function. Selenium deficiency is a particular problem, as the main dietary sources are plant-based food ingredients that must grow in selenium-rich, alkaline soils to accumulate the element. In many parts of the world soils are very low in selenium, and intakes in both the human and animal populations have declined alarmingly in recent decades, making the need for supplementation critical.
Inorganic sources of elements such as zinc can often meet the immediate needs of the animal, but have been found to be less well retained compared to organic sources of these metals. This is because animals have evolved mechanisms to extract organic forms from the diet, which are usually bound to amino acids (Surai, 2000).
An example of this is the organic selenium synthesised and stored in certain yeasts, which is primarily in the form of selenomethionine (as found in plant materials). This compound is readily absorbed and stored in the body, making it an effective source of selenium for synthesis of antioxidant enzymes during times of stress. Certain botanical ingredients have shown strong antioxidant properties, and are the focus of research in animal and human health as potential food and feed supplements. Hops, rosemary, thyme, turmeric and grapeseed extracts show interesting levels of antioxidant activity (Bagchi et al., 1998; Tucker, 2002).
Implications of oxidative stress for companion animals
Concerns regarding sources and effects of oxidative stress in companion animals center on cumulative effects associated with aging and age-related diseases. Cancer is a major killer of pet dogs and cats, primarily due to their long lives and exposure to oxidative stress, which can lead to the failure of correct DNA replication and tumour growth. Increases in oxidative stress can come from varying sources, and some examples are given in Table 2.
The majority of work concerning the relationship between oxidation, selenium and cancer has been conducted on humans, however the mechanisms are not species-specific. An excellent review of the relationship between cancer and selenium intake showed that incidence of cancer in vulnerable subjects (human and small animal studies) can be reduced by 30-50% (Whanger, 2004).
| Table 2. Major sources of oxidative stress in companion animals. |
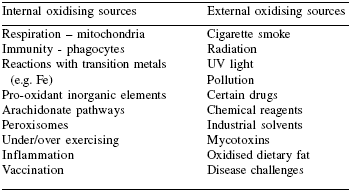 |
| Adapted from Furst, 1996. |
Oxidative effects are associated with inflammatory activity. An example of this is the antioxidant extract silymarin, which has been widely demonstrated to reduce arthritic and oedemal inflammation in both humans and rodents (Figure 1). Trials were conducted with mice whereby inflammation was introduced by latex injection into the ear. The width of the resulting inflamed area was then compared between groups of mice receiving various levels of the natural antioxidant (Gupta et al., 1999). Results indicated that inflammation caused by the latex injection was reduced by nearly 30% in mice fed 50 mg/kg antioxidant or more.
Reproductive organs are also sensitive to antioxidant status. Spermatozoa are rich in polyunsaturated fatty acids and require adequate membrane protection. Therefore, if antioxidant protection is compromised, reproductive success will be compromised as well. This effect has been well documented in agricultural species such as pigs and poultry. Trials conducted in poultry (Surai, 2002) have shown important reductions in lipid peroxidation in sperm from birds fed combinations of vitamin E and organic selenium (Sel-Plex®). Figure 2 shows that peroxidation levels in sperm decreased when males were fed increasing levels of vitamin E and organic selenium.
Very young animals are particularly vulnerable to stresses that can be regulated by antioxidant enzyme precursors. Selenium reserves in muscle are released under stress conditions for the synthesis of selenoprotein enzymes such as GSH-Px to prevent damaging effects of free radical overproduction from, e.g. immune stimulation through vaccination. Body stores of such compounds are especially important since many stresses are associated with decrease in food consumption. In contrast to inorganic selenium, the selenoamino acid from organic sources fed to the dam is transferred to milk and colostrum, ensuring nursing neonates obtain a continuing supply.
Trials on pigs have shown that feeding a suitable source of organic selenium (Sel-Plex®) to lactating animals can increase the levels of Se in milk, which is subsequently passed to the young. Furthermore this supplementation of selenium in the milk can then be used to generate stores in tissue such as liver. (Table 3, Figure 3).
The enzyme iodothyronine deiodinase, which is involved in thyroid hormone activation and influences basal metabolic rate and thermoregulation, is seleno-dependent. Young animals are less able to maintain body temperature, especially during disease challenge. Improving the capacity for tissue retention of selenium could potentially improve neonate livability.
| Table 3. Selenium content of tissues of neonatal piglets from sows receiving different selenium supplements. |
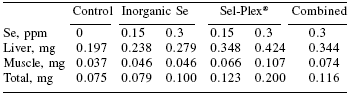 |
| Mahan, 2002. |
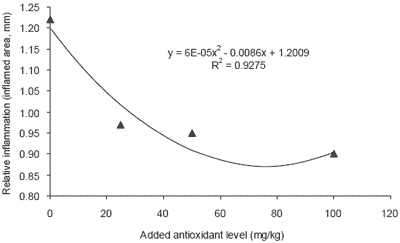 |
| Figure 1. Reduced inflammation in rodents supplemented with natural antioxidant sources (Gupta et al., 2000). |
 |
| Figure 2. Effect of Sel-Plex® and vitamin E supplementation on fresh sperm peroxidation (adapted from Surai, 2002). |
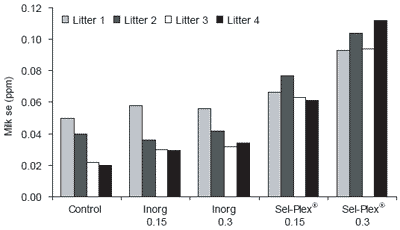 |
| Figure 3. Effect of selenium level (ppm) and form on sow milk selenium content (Mahan, 2002). |
Stability of pet food
A major use of antioxidants, especially those of a chemical nature, is as a food preservative to increase shelflife and ensure palatability. Recently there has been increasing interest in using more natural forms of antioxidants in pet foods – both as a marketing opportunity and to allay consumer worries regarding the use of chemicals.
The established use of meat and animal by-products in formulations, as well as the inclusion of polyunsaturated fats in premium pet foods, require specialised antioxidant programs to ensure food quality. The high levels of processing experienced, especially in extruded or canned foods, increase exposure to heat and air, promoting autooxidation reactions and facilitating rancidity. Any antioxidant, whether chemical, tocopherol-based or derived from natural plant sources, must be stable at high temperatures to be practical under such extreme conditions.
Losses of natural antioxidants from extrusion are frequently reported as being 50% or higher, making heat tolerance a key attribute for natural alternatives replacing synthetics. Research at the University of Santiago in Chile (Valenzuela, 2003) demonstrated that a commercial antioxidant system containing natural mixed tocopherols and rosemary extract (Nature Ban™) was thermally stable for 1 hr at 200°C and for 2 hrs at 150°C when tested on refined sardine oil.
Commercial experience indicates that certain natural antioxidants show less than 30% losses after extrusion. Figure 4 shows the comparative stability of chemical and natural antioxidants, including the commercial product Nature Ban™. Natural products show similar levels of protection as the chemical products when applied at similar doses. However a combination of natural antioxidants, as supplied via Nature Ban™, gave the longest stability period.
Comparisons between chemical and natural antioxidants for preventing rancidity in animal fats have also been conducted. Prevention of peroxide generation was monitored in chicken fat samples treated with chemical or natural antioxidants (Valenzuela, 2002). The results clearly showed that the combination of natural antioxidants in Nature Ban™ resulted in the lowest peroxide values over an extended storage period at high temperature. Such results demonstrate that the use of natural alternatives can ensure both the quality of pet food and the demands for more natural ingredients by purchasers.
Branding antioxidant benefits Branding and marketing pet food typically follows the same consumer decisions as for human food.
Recent trend analysis in retailing has shown that the growth market is for high quality and added value food (Hughes, 2003), so opportunities also exist for higher quality pet food formulated with functional ingredients such as antioxidants. As people realise the importance of antioxidants in their own diets, they increasingly look for similar benefits for their pets. An increase in public awareness regarding the importance of antioxidant-containing foods as well as mineral supplementation and ‘eating for health’ is already responsible for changes in many buying decisions.
A key factor in the marketing of antioxidantsupplemented pet foods is effective communication with the person making the purchase. The plethora of pet foods available is often bewildering, and, while it is well established that people are willing to pay for perceived higher quality, it is essential to put the message across regarding the added value antioxidants bring to the diet. This should be done in conjunction with making a bold statement via packaging that antioxidants are included.
Some manufacturers make claims for antioxidants based solely on the standard practise of adding vitamin E to the diet. Hence it is of key importance to use and promote the explicit benefits of well-established antioxidant products that add health and welfare benefits, backed up with proven science.
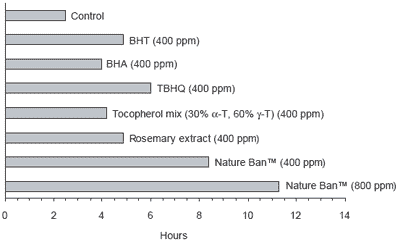 |
| Figure 4. Comparison of chemical and natural antioxidants on the induction period required for oxidation of highly refined fish oil under Rancimat test conditions. |
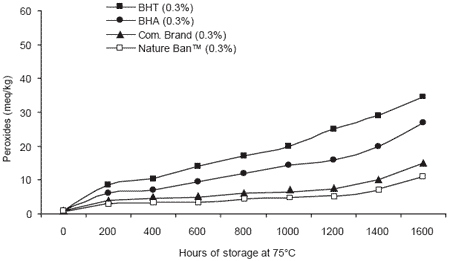 |
| Figure 5. Effect of chemical and natural antioxidants on peroxide generation in chicken fat (Valenzuela, 2002). |
by Lucy Tucker - Alltech Inc
This article hasn't been commented yet.


Write a comment
* = required field