Water activity as a tool for predicting and controlling the stability of pet foods
'Food safety' was one of many buzz phrases for 2005. As 2006 rolls forward, we can see that the attention focused on the safety of food is here to stay. The Federal Food, Drug, and Cosmetic Act requires that pet foods, like human foods, be pure and wholesome, containing no harmful or deleterious substances.
Consumers are becoming increasingly aware of the quality aspects of what they eat, as well as the quality of what they feed their beloved pets. One important aspect of food quality is the stability of the final product. Changes in physical, chemical or microbiological properties of food can be considered loss of stability. Water activity (Aw) is one of several important parameters that affect stability of foods.
Water activity is a measure of the free moisture in a foodstuff. It is also defined as the quotient of the water vapor pressure of the substance divided by the vapor pressure of pure water at the same temperature (US FDA, 2005). Free moisture can be explained as water that is available to participate in physical, chemical and biological reactions.
Water activity is not to be confused with moisture content. Moisture content is the combination of free and bound moisture. The relationship between moisture and water activity depends on the specific material. Silicon dioxide, for example, could absorb 50% moisture and maintain a very low water activity while crystalline sucrose absorbs little water until it reaches an Aw of 0.80. Characteristics of individual ingredients can be very important and will be discussed in this paper.
Role of water activity
MICROBIOLOGICAL CONTROL
Water activity plays a role in the microbial stability of ingredients and final pet foods. Bacteria, molds and yeast require water for growth; and every microorganism has a minimum water activity below which it will not grow. Table 1 details the level of water activity needed to inhibit different types of microorganisms (Fontana, 2000). A water activity of less than 0.85 is needed in food in order to avoid regulatory attention. At a water activity of less than 0.85, a food is considered non-hazardous because there is not enough available water to support pathogen growth.
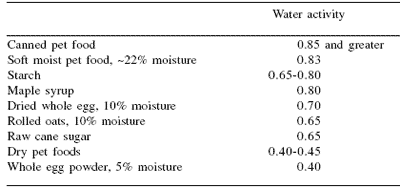
Water activities of many common ingredients and categories of pet foods are shown in Table 2. Dry pet food and hard treats typically are in the 0.40-0.45 Aw range. At this low level of available water (<0.60 Aw), microbial stability is not an issue. Canned foods, however, are typically higher than 0.85 Aw and must be treated as acidified foods. Water activities of less than 0.85, while considered non-hazardous from the standpoint of pathogenic bacterial growth, will still support the growth of many yeasts and molds.
Growth of these microorganisms can cause spoilage and physical deterioration. Soft pet foods and kibbles typically fall in this intermediate range (0.60-0.85 Aw). Supplementary processing such as pasteurization, pH control or addition of preservatives is necessary for protection of these foods. It often becomes a formulation tradeoff between maintaining a moist, chewy food and the added costs of preservatives and/or processing.
Table 1. Microorganisms and the water activities at which they are typically inhibited.

Fontana, 2000
Table 2. Water activities of some common foods and ingredients.
MOLD AND TOXINS
Mold can grow at water activity levels as low as 0.61. Types of mold, temperature and water activity play a role in determining growth characteristics. For example, Penicillium roqueforti germinated at 0.82 Aw at 25°C, 0.86 Aw at 30°C and was unable to germinate at 37°C. Eurotium repens germinated at 0.70 Aw at 30°C, but at 25 and 37°C, the minimum water activity for germination was 0.74. These fungi all grew faster under acidic rather than neutral pH conditions (Gock et al., 2003).
Toxins, notably mycotoxins in the case of feedstuffs, may be formed during the course of microbial growth, but not below 0.80 Aw (Lowe and Kershaw, 1995). Formation of mycotoxins depends on the type of mold, substrate and storage conditions, which include pH, temperature and water activity. A test of dry cereal-based pet foods and wild bird seed by Scudamore et al. (1997) for the presence of aflatoxin and ochratoxin found that 84% of these foods contained amounts that were below detectable limits. Ochratoxins were found in 10% of the samples at low levels. Penicillium, Eurotium and Aspergillus were the main genera of mold present on higher moisture samples of the food.
Mycotoxins can be formed on cereal grains such as corn and wheat. Processing temperatures can kill the mold but will not remove toxins that are already formed.
Testing of raw materials can help to limit the amount of toxins in food. Sampling can be very difficult because pockets of mold growth, and therefore mycotoxin formation, can occur during storage and transport. Mycotoxin formation can also occur during storage of the final food. Development of mycotoxins can be avoided by keeping the final water activity below 0.8.
INFESTATION
Insects are another potential problem during storage of pet foods, which in some cases can be controlled by water activity. Mites are commonly found in some food ingredients and have the ability to survive through some processing steps. Mites may be active at 5°C above 0.65 Aw, at 25° above 0.63 Aw and at 40°C above 0.60 Aw (Decagon, 2003). Dry pet foods and treats, which are well below 0.60 Aw, should remain free of mites.
CHEMICAL STABILITY
Water activity also influences chemical stability. Non-enzymatic browning, known as the Maillard reaction, is a chemical reaction between simple sugars and amino acids, which results in increasing color. As Figure 1 illustrates, non-enzymatic browning occurs between Aw 0.3 and 0.8. Browning leads to changes in appearance as well as to the formation of off-flavors. Enzymes can also lead to reactions in food, leading to off-flavors.
Enzyme reactions are substantially slowed at water activity levels of 0.8 or lower.
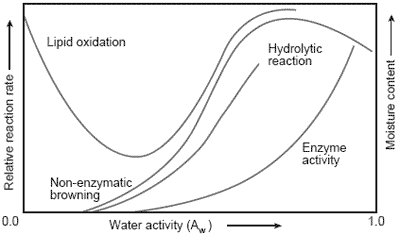
Figure 1. Reaction rates in foods as a function of water activity.
PHYSICAL STABILITY
Physical stability is extremely dependent on water activity. Dried foods will come to a final equilibrium with their environment. If the relative humidity of the environment is higher than the equilibrium water activity of the product, the product will take on water until it reaches equilibrium with the environment. If this new water activity is above the critical limit for the food, it will begin to cake, which is unacceptable to the consumer.
A critical water activity limit should be determined for each new product. This can be established by creating a water absorption isotherm (Figure 2). An isotherm is created at a set temperature by examining water activity readings vs. moisture readings for a product.
From the isotherm, the water moisture level that the product will reach at a specific relative humidity at a set temperature can be established. If the moisture level associated with the critical limit (physical characteristics change) for a product is noted, storage/packaging conditions can be established. A product with a critical limit above realistic storage conditions will not need special packaging. Products with a lower critical limit might need airtight sealed containers.
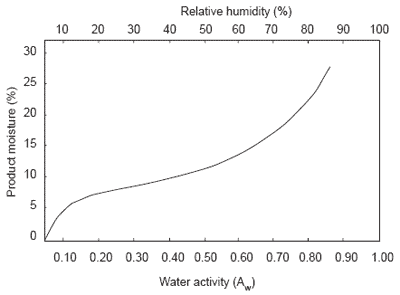
Figure 2. Typical water isotherm of a product.
Individual ingredients can also affect the final product stability. Ingredients with different initial water activities will come to equilibrium in the product. A spray-dried product with an initial 0.2 Aw mixed with an intermediate ingredient of 0.5 Aw will reach equilibrium at a value between the two. The higher activity ingredient will lose available water to the lower activity ingredient. The characteristics of each ingredient could change.
Again, this depends on the critical limits of each individual ingredient, as well as the critical limit of the final product. Salwin (1963) developed an equation using water activity and moisture from individual component isotherms to predict the water activity of the mixture at equilibrium (Figure 3). In order for the prediction to represent the actual situation, isotherms must be developed at the temperature at which the final product will be held. Packaging should be such that the environmental humidity need not be considered a factor.

Figure 3. Salwin equation for predicting water activity at equilibrium.
It is typical for some pet foods/treats to have a mixture of soft chewy pieces and some harder pieces. The harder pieces are considered valuable for their ability to keep teeth cleaned, while the softer pieces are considered more palatable. When mixed, the two materials will come to equilibrium.
In order for this mix to remain stable, the individual components and final product must be developed in order that the final equilibrium water activity will allow the two components to retain their original characteristics.
This has been the subject of many patents on both the pet food and human food sides.
Specialty packaging, processing and ingredients have all been used in order to obtain the goal of maintaining a stable final product.
Transfer of water between ingredients is a common cause of caking. The transfer of water from an ingredient with higher water activity to an ingredient with a lower water activity causes one of the ingredients to reach its critical limit, initiating caking. Storage factors such as temperature can change the rate of this reaction. Lower temperatures will slow the reaction, while higher temperatures speed reaction rate.
Product development
Food technologists will utilize all of the information above when developing a new product. The first goal is to establish the type of product (low, intermediate, or high moisture) to be produced. Producers of a low moisture food will not need to worry about microbiological issues. Water activity will be a factor in this food with regard to physical characteristics. The Salwin equation in Figure 3 is useful in predicting the final water activity of a mixture of ingredients. Addition of ingredients such as salt, sugar, and silica can help adjust the final water activity to a desired endpoint.
An intermediate food is considered safe with regard to pathogen contamination, but is subject to spoilage organisms, enzymatic and non-enzymatic breakdown and physical changes. The US Code of Federal Regulations (21 CFR 110.80 (14)) states that intermediate foods that rely on the control of water activity for preventing growth of undesirable microorganisms shall be processed to, and maintained at, a safe moisture level.
Monitoring water activity, adjusting the soluble solids:water ratio or controlling packaging can accomplish this. Further drying, or use of solutes such as salt, sucrose or sorbitol could be used to lower the water activity; however this might not produce the desired final product characteristics.
Preservatives such as sodium benzoate, propionic acid and potassium sorbate can be used to control microbial growth. Factors such as pH, target microorganisms, taste and cost can be used to decide on the appropriate preservative. Water isotherms should be used to predict the physical stability of the product, taking critical limits for desired characteristics into account.
High moisture foods (>0.85 Aw), such as canned pet foods, must be processed in conformance with low acid canned food regulations (21 CFR, Part 113). These regulations state that critical factors such as water activity must be used in conjunction with thermal processing.
Conclusion
In order to produce a pet food that is both commercially viable and safe, water activity must be used as both a development tool and a quality assurance mechanism.
Water activity values are an extremely important consideration in any type of pet food, since water activity plays a role in the physical, chemical and biological stability of the product.
Several methods have been given for evaluating and determining the effect of water activity on a particular formula.
The challenge to the pet food industry is to use the right combination of ingredients, processing and packaging to create products that meet safety and quality standards, meet cost parameters and still maintain integrity of design to satisfy consumers.
by R.A. Timmons, North American Biosciences Center, Alltech Inc
This article hasn't been commented yet.


Write a comment
* = required field